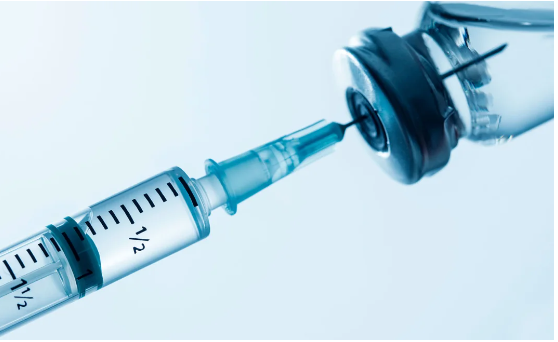
Poison control centers throughout the United States are witnessing a significant surge in calls related to semaglutide, an injectable medication utilized for diabetes and weight loss. Some individuals are reporting symptoms linked to unintentional overdoses, resulting in severe nausea, vomiting, and stomach pain, necessitating hospitalization. Fortunately, cases appear to have resolved after patients received intravenous fluids and medications to manage nausea.
READ: Delta Epic 24-Hour Flight Nightmare! Unbelievable Twists, Sharp Turns, and a Reunion for the Ages!”
From January to November, America’s Poison Centers reported almost 3,000 calls involving semaglutide, marking a more than 15-fold increase since 2019. In 94% of cases, semaglutide was the sole reported substance. Dr. Kait Brown, clinical managing director of the association, highlighted that most calls involved dosing errors, often accidental double doses or incorrect doses.
Poison
Semaglutide, approved by the FDA in 2017 and sold as Ozempic for diabetes and Wegovy for weight loss, can cause stomach and bowel side effects, even when used as directed. The rise in demand, particularly after celebrities endorsed Ozempic for weight loss on social media, led to shortages, prompting some qualified pharmacies to produce compounded versions. These versions, however, may differ from the patented drug, containing unapproved doses or semaglutide salts not tested for safety.
Compounded versions, which may be more affordable, gained popularity despite FDA warnings against their use. Poison control centers are receiving increased calls, and reported symptoms make it challenging to determine if they stem from patented drugs or compounded versions. Dr. Joseph Lambson highlighted cases of people mistakenly taking significantly higher doses of compounded semaglutide, citing the ease of errors with multidose glass vials compared to pre-filled pens used for name-brand drugs.
The FDA’s efforts to halt the sale of knock-off versions and Novo Nordisk’s legal action against sellers underscore the risks associated with compounded semaglutide. Poison centers are receiving calls from adults aged 40 to 70, with dosing errors reported for both compounded forms and prescription pens. Patient safety remains a priority, and emergency care for semaglutide overdoses involves supportive measures, as there is no specific antidote. Symptoms of low blood sugar should be monitored, especially when combined with other diabetes medications.




