A groundbreaking study BCMA-CD19 led by Professor Junnian Zheng and Ming Shi from the Cancer Institute of Xuzhou Medical University, along with Professor Guiyun Cui and Wei Zhang from the Affiliated Hospital of Xuzhou Medical University, has reported for the first time the use of BCMA-CD19 bispecific CAR T cells for treating relapsed/refractory Chronic Inflammatory Demyelinating Polyneuropathy (CIDP).
Understanding BCMA-CD19 and Current Treatments
Chronic Inflammatory Demyelinating Polyneuropathy (CIDP) is a rare neurological disorder characterized by sudden onset symptoms, including nerve damage affecting movement, sensation, speech, breathing, and heart rate. Over 80% of patients experience muscle weakness, impaired gait, absent tendon reflexes, sensory loss, balance problems, and in severe cases, paralysis, irregular heart rhythm, and difficulty breathing. Current treatments, such as glucocorticoids, plasma exchange, and intravenous gamma globulin (IVIg), manage symptoms but cannot fully eradicate the disease.
The Promise of CAR-T Cell Therapy
In recent years, chimeric antigen receptor (CAR)-T cell therapy has shown remarkable efficacy in treating hematologic tumors and autoimmune diseases such as systemic lupus erythematosus. This study explores the use of BCMA-CD19 bispecific CAR T cells for treating relapsed/refractory CIDP.
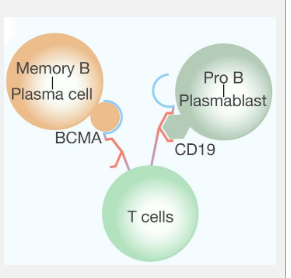
Innovative Approach with BCMA-CD19 Bispecific CAR T Cells
B cell clearance, often coupled with anti-CD20 antibodies, has been a treatment for CIDP. However, CD20 and CD19 are primarily present in early B cell development stages and are absent in long-lived plasma cells. By leveraging the BCMA protein’s presence in plasma blasts and long-lived plasma cells, researchers designed bispecific CAR-T cells targeting both CD19 and BCMA. This approach aims to reset immune responses by temporarily and deeply eradicating B cells and plasma cells.
Case Study: Remarkable Recovery
A 44-year-old man with relapsed/refractory CIDP exhibited distal limb numbness and weakness. Following the 2021 European Society of Neurology/Society of Peripheral Neurology CIDP Guidelines, he was diagnosed with distal CIDP without IgG4 autoantibodies. After an evaluation, he was deemed eligible for bispecific CAR-T therapy for autoimmune disease, which he successfully underwent.
Post-therapy, the patient showed significant improvement according to INCAT disability and MRC scores. Remarkably, nearly full muscle power recovery was observed 180 days after CAR-T administration, enabling him to walk again. Initially, it took him 21 seconds to cover a 10-meter walk, but by day 180, he managed it in just 13 seconds. Electrophysiological assessments showed significant improvement in the median, ulnar, common peroneal, and tibial nerves. After the initial 180-day follow-up, he was monitored every 90 days for potential relapse. Remarkably, for over a year, the patient discontinued all immunosuppressants without disease recurrence, and the presence of GM4 and GD3 antibodies continued to diminish three months post-CAR-T cell therapy.
Safety and Tolerability
Regarding safety, the patient developed a fever (38-39°C) and transient IL-6 elevation 6-14 days after CAR-T cell therapy, which was managed with acetaminophen. He also experienced hypotension (86-97/35-59 mmHg, grade 2) 1-15 days post-infusion, recovering after two weeks of bed rest and hydration. No other toxicity associated with CAR-T cell therapy was observed.
Implications and Future Prospects
This case demonstrates the viability, tolerability, and effectiveness of BCMA-CD19 bispecific CAR T cells for treating stubborn/repetitive CIDP. Even without continued immunosuppressants, remission persisted despite rising B cell levels. This approach has the potential to aid those suffering from autoimmune nerve disorders linked to B cells, such as neuromyelitis and myasthenia gravis.
The study highlights the change in patient symptoms post-treatment and affirms the safety of CAR-T cell therapy for CIDP. While this is a single case report, more extensive studies and extended follow-up, which are underway, will add significant clinical value. This “dual-target” strategy represents a promising step toward creating a potentially curative treatment for CIDP.




